What Is a Solid?
A solid is a phase of matter characterized by a fixed shape and a fixed volume. In solids, particles are packed closely together and held in place by strong attractive forces. These particles are not completely motionless, but they vibrate around fixed positions. Because of this tight arrangement, solids do not flow like liquids or expand to fill a container like gases. Common examples of solids include ice, wood, metal, and rock, all of which maintain their shape unless an external force is applied.

Particle Arrangement and Movement in Solids
The particles in a solid are arranged in a structured way that limits their movement. In many solids, especially crystalline solids like salt or ice, particles form repeating patterns called lattices. This orderly arrangement gives solids their strength and rigidity. Even though particles vibrate due to thermal energy, they do not have enough energy to move freely past one another. As temperature increases, the vibrations become stronger, which can eventually lead to a phase change if the solid absorbs enough energy.
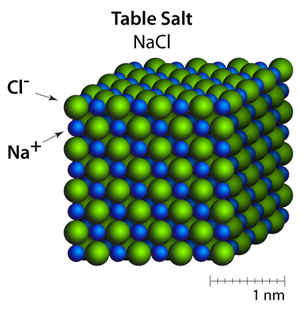
Types of Solids
Solids can be grouped into different categories based on their internal structure. Crystalline solids have a highly organized particle arrangement and include substances such as sugar, quartz, and most metals. Amorphous solids lack a regular pattern and have particles arranged more randomly; examples include glass, plastic, and rubber. Some solids are also classified by their properties, such as conductors, insulators, or magnetic solids. These differences affect how solids respond to heat, electricity, and physical stress.

How Solids Change Phase
Solids change phase when they gain or lose energy. When a solid absorbs enough heat, the particles vibrate more intensely until they overcome the forces holding them in place, causing the solid to melt into a liquid. This occurs at a specific temperature known as the melting point. Some solids can skip the liquid phase entirely and turn directly into a gas through sublimation, such as dry ice. When energy is removed from a liquid, particles slow down and lock into position, forming a solid through freezing.

Importance of Solids in Everyday Life
Solids play a crucial role in everyday life and human civilization. Buildings, tools, and machines are made from solid materials chosen for their strength and durability. Many everyday objects, from clothing to electronics, rely on the properties of solids to function properly. In nature, solids such as rocks and ice shape landscapes and ecosystems. Understanding solids helps scientists and engineers design safer structures, develop new materials, and improve technology used in daily life.
